Colloquially speaking, the term “rubber” can refer to many different things. On the market, we have tires, erasers, rubber bands, gum arabic, and chewing gum. Generally speaking, rubber is a substance that finds application in a wide variety of products – from mousepad material and swimming caps to rubber hoses, seals, wiper blades, rubber sheets, and rubber floor coverings.

Rubber has been known and widely used by humans for over 1,000 years. Historically, rubber products were made from naturally occurring materials. Nowadays, the vast majority of rubber products we encounter are made from synthetic rubber, produced in chemical plants. The main reason is that the demand for natural rubber far exceeds the ability to obtain it, or as some say, to produce it. The continually high demand for rubber stems from its fantastically versatile and useful nature! We invite you to learn about one of the most fascinating substances in the world.
What is Rubber?
People rarely specify what type of rubber they are referring to when using the term. There is a broad spectrum of different kinds of rubber. The primary and basic division is into two main types: natural rubber (plant-derived) and synthetic rubber (an elastomer produced artificially in a lab). Commercially, the most popular types of rubber are styrene-butadiene rubber, polyacrylates, polyvinyl acetates (PVA), vinyl chlorides (PVC), and polychloroprene (better known as neoprene). Despite natural and synthetic rubbers being very similar in appearance, they differ chemically and are products of entirely different production processes.

Natural Rubber
Natural rubber is obtained from a white, milky fluid known as latex, which is collected from certain types of plants after making incisions. For example, common dandelions produce small amounts of latex when their stems are broken, from which natural rubber could theoretically be extracted – though this process would be very inefficient as it would require growing a vast number of dandelions to obtain a small amount of rubber. Although humans know of 200 different plant species that produce latex, 99 percent of the world’s natural rubber production comes from the latex of a tree species known as the Brazilian rubber tree (Hevea brasiliensis).
Bulk Mouse Pad Material
Latex from this tree is composed of one-third water and one-third rubber particles in a colloidal suspension. Natural rubber is an isoprene polymer with the chemical formula C5H8. Simply put, it consists of many thousands of units of C5H8 (isoprene monomer), which form long tangled chains. These molecular chains can be easily stretched and untangled, but they also easily return to their original shape. This property makes rubber elastic.

Synthetic Rubber
Synthetic rubbers are produced in chemical plants, using petroleum-based substances as the base. One of the best-known and still most popular types is neoprene (the trade name for polychloroprene), which results from the reaction of acetylene and hydrochloric acid. Another very popular type of synthetic rubber is butadiene-styrene rubber (SBR), from which rubber sheets available in our store are made.

How is Rubber Made?
In 1839, American inventor Charles Goodyear developed the vulcanization process (heat treatment), which hardens and strengthens rubber. He spent a long time researching how to turn natural rubber into a useful product. His discovery was somewhat accidental. When he accidentally dropped a piece of rubber onto a hot stove, it began to transform into a black, vulcanized form, more similar to the rubber we commonly know today. Although he managed to invent one of the most useful materials, Goodyear never significantly enriched himself from his discovery and died facing financial difficulties. However, his name lives on thanks to the popular tire company named in his honor, acknowledging his work and great achievement.
The production of rubber from natural rubber consists of several distinct processes. The first is obtaining latex from rubber trees. This involves making a series of incisions in the tree bark in a V shape, from which latex oozes. It is collected into containers attached to the trunk. Latex collected from many trees is then mixed and washed with acid to achieve coagulation (clumping of particles). The rubber produced in this way is then formed into blocks or sheets and dried before further processing.
The rubber obtained in this manner is raw rubber, which in itself is not very valuable. It crumbles at lower temperatures, releases an unpleasant odor, and becomes sticky at higher temperatures. Therefore, further processing is necessary to improve its properties. The first process is mastication, which involves mechanically treating the rubber using presses or rollers to plasticize and soften it. After mastication, chemical enhancers (improving properties like aging resistance) are added to the rubber. The rubber then undergoes calendering – a process of repeatedly shaping the rubber using sets of rollers, under whose pressure it plasticizes. The final stage of the process is vulcanization – sulfur is added, and the rubber is heated to about 140 degrees Celsius.
Where Does Rubber Come From?
As the name suggests, the Hevea brasiliensis rubber tree comes from Brazil, from where its seedlings were taken and brought to countries in the Far East such as Malaysia, Indonesia, Burma, Cambodia, China, and Vietnam. The Southeast Asian region traditionally remains the largest producer of natural rubber. During World War II, supplies of rubber from this part of the world to the West were limited, and the demand for rubber products for the military was very high. This significantly accelerated the work on synthetic rubber in Germany and the United States. Today, most natural rubber still comes from Far Eastern countries, while France, Germany, and Russia lead in synthetic rubber production. The world’s largest natural rubber plantation is the Harbel Plantation near Monrovia in Liberia, established in 1926 by Firestone company founder Harvey S. Firestone and his wife Isabella Bell.

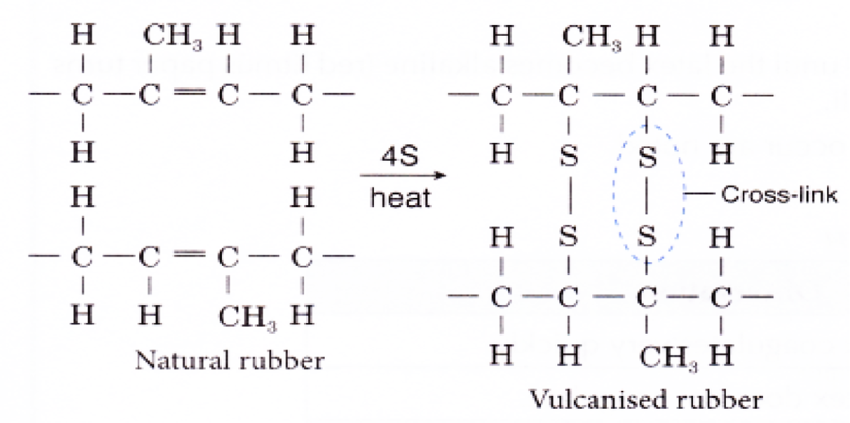
How Does Vulcanization Strengthen Rubber?
Rubber in the form it is obtained from the tree is very fluid latex. Even after some time, natural rubber coagulates; it is spongy, has an unpleasant odor, and is not particularly useful. However, the rubber we encounter in products like tires, seals, mats, or rubber sheets is vulcanized rubber – boiled with sulfur and other additives, making it harder, stronger, and more resistant to aging.
What causes such differences between raw and vulcanized rubber? In its raw state, rubber consists of molecules in long, very loosely and weakly connected chains. It is relatively easy to stretch and separate them – that’s why latex is so elastic. During vulcanization, additional sulfur atoms help create bonds between the rubber molecules, a process known as cross-linking. These bonds act like trusses on a bridge – stiffening and strengthening the molecular structure of the rubber.
What Do We Use Rubber For?
The physical and chemical properties of the material translate into how we use it. Even if we didn’t know the rubber production process, considering its properties, we could come up with its applications. Everyone knows that rubber is strong, stretchy, elastic, durable, and waterproof. No wonder it is used to produce mouse pads,waterproof clothing, boots, hydraulic hoses, or seals.

The most popular use of rubber is in the tire industry. About half of the world’s rubber production is used to make car and truck tires. Rubber is used for both the hard outer tires and the inner tubes inside them. The tubes are made of a slightly different material – butyl rubber, which is highly impermeable and effectively retains gases. This keeps truck tires inflated for a long time.
what are mousepads made of?
The ability to obtain both hard and soft rubber significantly expands the range of its potential applications. Soft and elastic latex is used in a wide range of everyday products, from desk mats,boob mouse pad,erasers and balloons to condoms and protective gloves.

Hard rubber is used for more demanding applications, such as roofing membranes, garden path and pond linings, or inflatable boat production. Since rubber is a flexible and strong material, but also a very poor conductor with good thermal insulation properties, rubber sheets are often used as a solid yet thin protective material for cables, fiber optics, and heat pipes. EPDM rubber sheets, weather-resistant, are also used as construction material, such as rubber pads under terraces or attic insulation. Rubber sheets are also excellent soundproofing material.
The global appetite for rubber is enormous. At the same time, the world continues to produce a lot of rubber waste, posing an ecological threat. A large part consists of used car tires, which themselves then become an important raw material. Some used tires are re-treaded, especially truck and other vehicle tires. Another part is ground into fine granules, which are pressed into rubber granulate sheets. These sheets are used as flooring for facilities like safe playgrounds. Unfortunately, more than half of used tires still end up in landfills. Rubber producers are continually working on new methods of recycling tires. Increasingly, old tires find new life as shoe soles, or other rubber car components.
A Few Historical Facts About Rubber
- 1000 BCE: Indigenous people in Central and South America learned to make waterproof clothing by impregnating it with latex from rubber trees.
- 1731: During an expedition to South America, French traveler Charles Marie de La Condamine sends rubber samples to Europe, sparking scientific interest.
- 1770: English scientist Joseph Priestley, who discovered oxygen, finds that a piece of rubber can erase pencil marks from paper, hence the eraser.
- 1791: Englishman Samuel Peal develops a method of waterproofing fabric with a rubber solution.
- 1818: Scottish medical student James Syme uses rubberized fabric to produce raincoats.
- 1823: Scotsman Charles Macintosh learns of James Syme’s work, refines it, and patents it, gaining fame and fortune from the popularity of rubber raincoats.
- 1839: American inventor Charles Goodyear accidentally discovers rubber vulcanization, initiating work on new rubber types.
- 1909: German scientist Fritz Hofmann develops the first synthetic rubber.
- 1912: American chemist and Nobel Prize laureate Wallace Hume Carothers, known for his contributions to the invention of nylon, patents synthetic rubber neoprene (polychloroprene).
These are just a few facts, but they should give you an idea of how rich and extensive the history of rubber production is. We hope you found this brief history and overview of rubber types, production processes, and properties useful and interesting. Perhaps you will now appreciate the contribution of this natural yet advanced product of chemical synthesis in various industries and our everyday lives.

